Introduction: Data on lymphoma patients with SARS-CoV-2 infection (COVID-19) are lacking. A large cancer cohort study showed that number of comorbidities, active cancer, and worse performance status were associated with increased 30-day mortality (Kuderer 2020). Variables contributing to risk of poor outcomes of COVID-19 in lymphoma patients have not been reported. We aimed to investigate the effect of COVID-19 on lymphoma patients' clinical outcomes including the effect of lymphoma-specific therapies on COVID-19 recovery.
Methods: We conducted a multicenter, retrospective, observational cohort study. Data was obtained from 4,302 patients flagged in the electronic health records system with COVID-19, of which 89 lymphoma patients were identified. Presenting clinical information was abstracted from review of unstructured notes as well as structured data. Demographic and baseline parameters were summarized. The differences in the distributions between CD20 antibody treated or non-treated patients were compared using Mann-Whitney's test for continuous variables and Pearson's chi-squared test for categorical variables. The Kaplan-Meier method was utilized to compare the median time from date of diagnosis to date of last follow up or death.
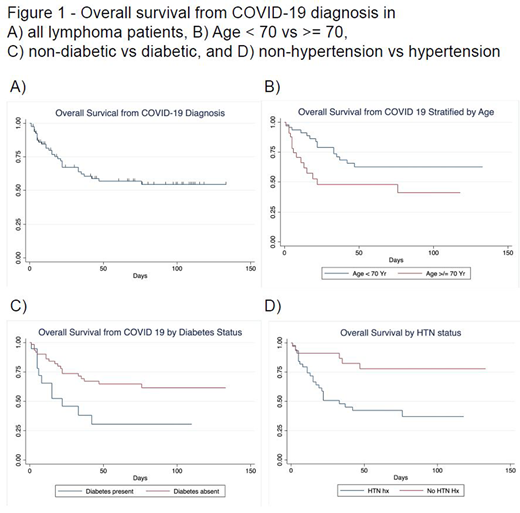
Results: 89 lymphoma patients were evaluable. The median age was 67 (range 29-94) with a male predominance (63%). The most common presenting symptoms were fever (54%), shortness of breath (52%), cough (47%), and gastrointestinal (13%). Hypertension and diabetes were present in 51 of 89 (57%) and 19 of 89 (21%) patients, respectively. Laboratory abnormalities included a median absolute neutrophil count of 4.67x103/mcL (range 0-15.9) and lymphocyte count 0.8x103/mcL (range 0-97.7). Inflammatory markers at diagnosis showed median CRP 9.4 mg/dL (0.7-126, n=62) and ferritin of 966 ng/mL (96-14,511, n=42). 76 of 89 (85%) patients required hospitalization, 11 (12%) required mechanical ventilation, and 31 (Case Fatality Rate=35%) died. The overall survival of lymphoma patients is shown in Figure 1A. The presence of hypertension (HR 3.7, p=.005), diabetes (HR 2.6, p=.02), or advanced age ≥70 (HR 2.2, p=.03) were associated with increased COVID-19 related mortality (Figure 1B-D).
Lymphoma subtypes included CLL (n=32), DLBCL or Burkitt's (n=18), indolent non-Hodgkin (n=20), Hodgkin (n=6), T cell lymphoma (n=6), and other (n=7). 76% were stage III-IV (n=68), with 40 patients (44%) on active lymphoma treatment at the time of COVID-19 diagnosis. 41 patients (48%) received rituximab within the last 3 years, with 11 requiring chronic immune gamma globulin replacement. 25 patients were tested for seropositivity to COVID-19 at median of 42 days from COVID-19 diagnosis (IQR 32-50). 5 of 9 (55%) patients who did not receive CD20 antibody therapy showed immunity. In contrast, out of 16 patients who received anti-CD20 treatment and had a COVID-19 IGG antibody test, only 2 (12.5%) became seropositive (p =.03). Patients who tested seronegative in the CD20 antibody group and had a second negative IgG antibody test (n=6) were a median of 50 days (range 12-82 days) from COVID-19 diagnosis.
The median time from diagnosis until a negative COVID-19 PCR test in CD20 antibody treated patients was 56 days (IQR 29-67.5) compared to 14 days (IQR 13-54) (p=0.037) in CD20 antibody naïve patients.
Conclusion: Survival was poor in lymphoma patients with hypertension, diabetes, or age ≥ 70. The rate of seropositivity in patients treated with CD20 antibody therapy was significantly lower (12.5% vs 55%). Patients exposed to anti-CD20 therapy also required significantly more days to clear viral shedding (median 56 days vs 14 days). While limited by sample size, this study merits further validation and suggests anti-CD20 therapy may place lymphoma patients at higher risk of prolonged or repeated COVID-19 infection. Further investigations to determine infectivity of patients with prolonged SARS-CoV2 shedding are needed.
Mato:Janssen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding. Leslie:Celgene/BMS: Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics/Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Speakers Bureau; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; KitePharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Speakers Bureau. Goy:Constellation: Research Funding; Genentech/Roche: Research Funding; Infinity: Research Funding; Karyopharm: Research Funding; Morphosys: Research Funding; AbbVie: Research Funding; MD Anderson: Research Funding; Regional Cancer Care Associates/OMI: Current Employment; Infinity Verastem: Research Funding; Xcenda: Consultancy; Hackensack UMC and University of Nebraska: Research Funding; COTA: Consultancy, Current equity holder in publicly-traded company, Other: leadership role; Kite, a Gilead Company: Consultancy, Current equity holder in publicly-traded company, Honoraria, Other: leadership role, Research Funding; Janssen: Consultancy, Honoraria, Other: leadership role, Research Funding; Celgene: Honoraria, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: leadership role, Research Funding; Acerta: Consultancy, Honoraria, Other: leadership role, Research Funding; RCCA/OMI: Current Employment; PracticeUpdate Oncology: Consultancy; Bayer: Research Funding; CALBG: Research Funding. Feldman:Kyowa Kirin: Consultancy, Research Funding; Celgene: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Portola: Research Funding; Takeda: Honoraria, Other: Travel expenses; Seattle Genetics, Inc.: Consultancy, Honoraria, Other: Travel expenses, Research Funding, Speakers Bureau; Janssen: Speakers Bureau; AstraZeneca: Consultancy; Corvus: Research Funding; Rhizen: Research Funding; Pfizer: Research Funding; Kite: Honoraria, Other: Travel expenses, Speakers Bureau; Viracta: Research Funding; Trillium: Research Funding; Bayer: Consultancy, Honoraria; Pharmacyclics: Honoraria, Other, Speakers Bureau; Amgen: Research Funding; Eisai: Research Funding; Cell Medica: Research Funding; Abbvie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.